Research Ethics
Aiming to protect the rights, welfare and safety of human participants and animal subjects in research, the University requires all research projects to obtain prior ethical and/or safety approval. The governance structure and policies relating to research ethics are as follows:
Policies
The University adopted the following sets of research ethics policies and procedures which underpin the requirements and expectations for conducting responsible research. The policies also define the action to be taken in the event that an individual is suspected or accused of research misconduct.
- Policy on Responsible Conduct of Research
- Standard Operating Procedures for Human Clinical Research
- Operational Guidelines and Procedures for Human (Non-Clinical) Research Ethics Panel
The Governance Structure
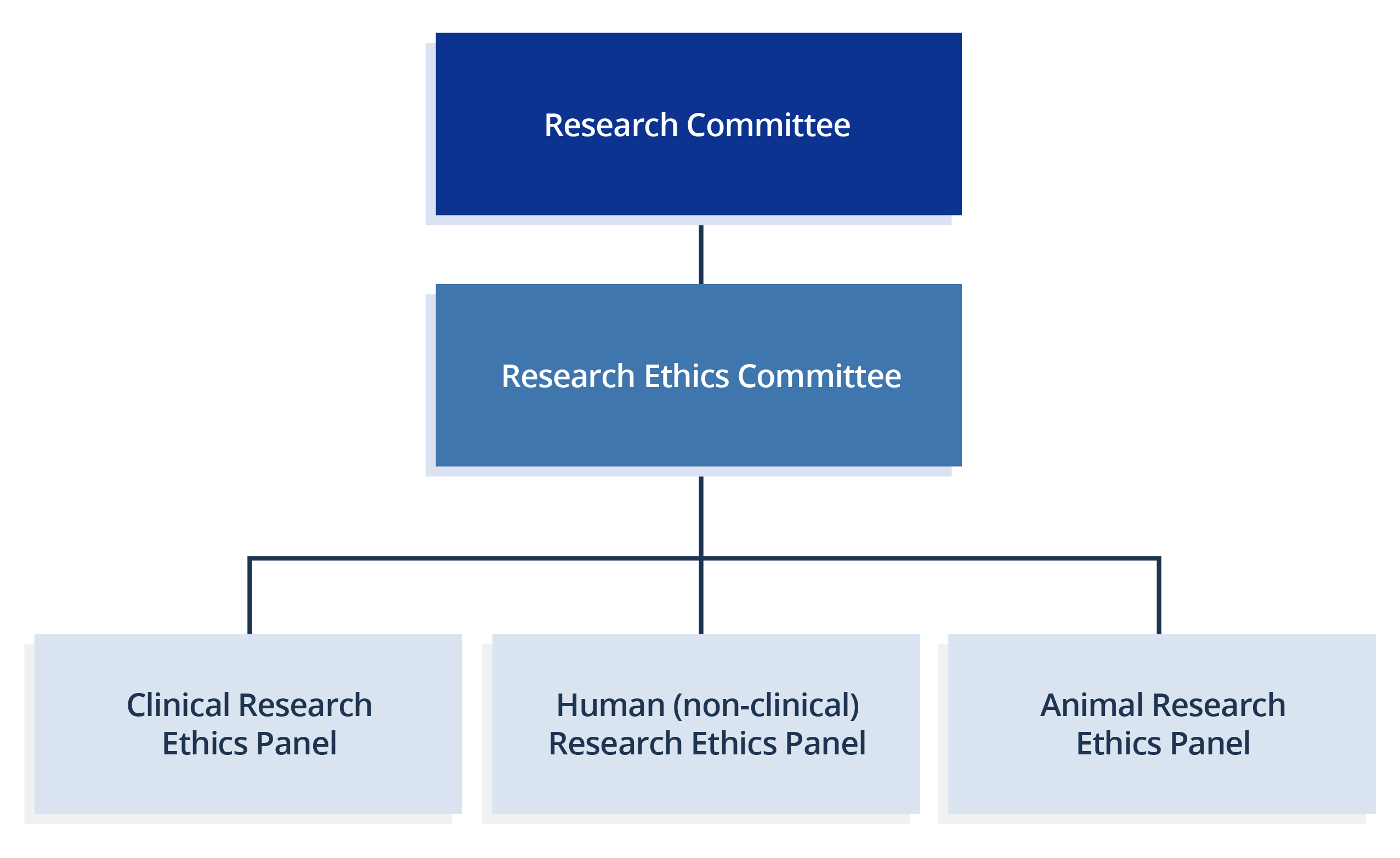

Research Ethics Committee (REC) and the three panels
The REC primarily performs the functions of policy review and development relating to research ethics, as well as the review of complaints and appeals. For handling applications of research ethics, three panels are formed:
- The Clinical Research Ethics Panel;
- Human (non-clinical) Research Ethics Panel; and
- Animal Research Ethics Panel
Ethics Application Procedures
The three panels handle all ethics/safety applications on a centralized and rolling basis, and help facilitate the ethics clearance within a reasonable timeframe.
For more details, please refer to the Research Ethics [Require SSOid login]